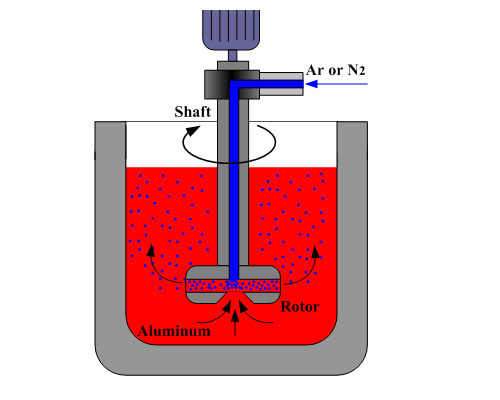
Degassing with fluxing gas can remove the hydrogen and inclusion in molten aluminum. Referring to the fluxing gases, what is contemplated is that a non-reactive gas, preferably along with a reactive chlorinaceous or other suitable halogen gas, be bubbled through the molten aluminum in the degassing chamber. The chlorinaceous gas where employed may be a vaporous chloride such as aluminum chloride or hexachloroethane or any suitable source of active chlorine although chlorine is preferred because of availability and ease of handling, is provided in conjunction with a non-reactive gas flux. While a chlorinaceous gas, especially chlorine, is preferred, other halide or halogen gases suitable in fluxing molten aluminum can also be utilized. These gases include boron tetrafluoride and fluorine but these are less preferred than the chlorinaceous gas since they present health hazards.
The non-reactive gas flux may be any of those commonly designated as inert gases such as helium, neon, argon, krypton, xenon, and mixtures thereof although argon is preferred for reasons of cost and availability. Also, nitrogen or carbon dioxide can be employed as the non-reactive gas provided that proper measures are taken to avoid the formation of carbides or nitrides.
While, particularly where the charge is relatively clean, the non-reactive fluxing gas alone can remove the TiO2 and SiO2 and CaO impurities, it is preferred to also use the chlorinaceous or other halogen gas which furthers the fluxing action and also improves the removal of other contaminants.
Degassing with fluxing gas adopts mixed gas, a mixture of 2 to 10 percent chlorine in argon is quite suitable. The gases are preferably blended before introduction to the degassing chamber. That is, premixing the respective gases and introducing that mixture through all of the dispersers is considerably preferable to introducing the halogen or chlorinaceous gas through some dispersers and the non-reactive gas through other dispersers. In general, the total amount of fluxing gas is related to the amount of metal being treated. Both the amount of the gas and the proportion of chlorine are related to the character of the scrap to be melted, the higher levels of dirt or contamination being accorded higher amounts of fluxing gas and richer chlorine levels in that gas.