Degassing aluminum with nitrogen is an economical degassing method. In aluminum foundry, it is a common practice to degass molten aluminum with argon gas. It is an effective method to remove hydrogen in metal by using this rare gas, which will not adversely affect the metallurgical quality of the obtained metal. On the other hand, more and more manufacturers are looking for cost reduction opportunities, and nitrogen can usually replace argon instead of molten aluminum.
When casting aluminum, impurities called inclusions can produce weak spots in the product. One of the most common causes of these impurities is the presence of hydrogen in molten aluminum due to atmospheric humidity, combustion in gas furnaces, condensates on tools, fluxes, and alloy additives.
Hydrogen is soluble in liquid aluminum and can pass almost as easily as through the air. As the liquid metal cools and hardens, hydrogen flows from the high-pressure region to the low-pressure region, forming cavitation. When the metal solidifies, these cavities become inclusions and weak points in the casting.
The demand for high-quality aluminum products is increasing, especially in cutting-edge industries such as aerospace. Degassing is a process used to remove hydrogen from molten aluminum. The most popular degassing method involves introducing nitrogen into liquid aluminum. The hydrogen that causes these inclusions is simply drawn into the nitrogen bubbles, then carried to the surface and released, thus eliminating the hydrogen that causes the impurities.
The technology of removing impurities from the melt is becoming more and more important for foundry and widely used in the industry. One of the most effective technologies used by foundry is rotary degassing.
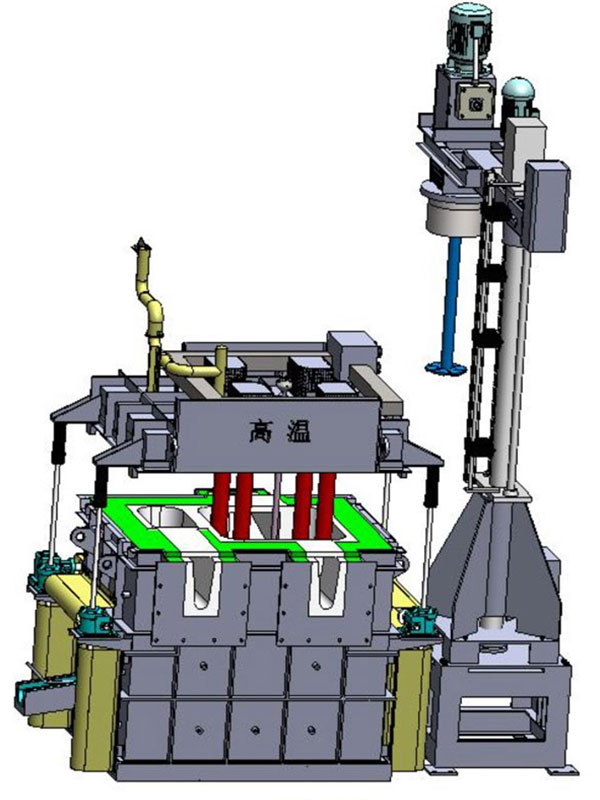
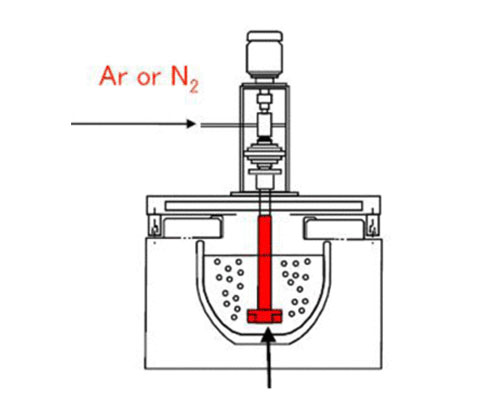
Rotating degassing system removes these undesirable components by bubbling gas, usually nitrogen, through molten metal. The gas is usually introduced through a degassing rotor, which reduces the bubble size and disperses nitrogen throughout the molten metal bath. As the resulting bubbles rise through the molten metal mass, they absorb the hydrogen dissolved in the metal and remove it from the melt. In addition, the non-metallic solid particles are swept to the surface to be removed by skimming.